orbital diagram arsenic
So the next six electrons will enter the 4p orbital just like the 3p orbital. We can also find the valency of oxygen with the help of the periodic table.

Webelements Periodic Table Arsenic Properties Of Free Atoms
The pages and hyperlinks of the World-Wide Web may be viewed as nodes and edges in a directed graph.

. Orbital Diagram of All Elements Diagrams given Inside. Filling begins with the lowest energy orbital which is the 1s obital. This is clearly shown in the figure of the orbital diagram of rubidium.
The six commonly recognised metalloids are. The four electrons form perfect covalent bonds with four neighboring atoms. Which has been discussed in detail above.
The Bohr Model of ChlorineCl has a nucleus that contains 18 neutrons and 17 protons. PhosphorusP arsenicAs antimonySb bismuthBi and moscoviumMc. This is followed by the 2s.
Silicon sits next to aluminum and below carbon in the periodic table. To write the orbital diagram of fluorineF you have to do the electron configuration of fluorine. This nucleus is surrounded by three-electron shells named K-shell L-shell and M-shell.
Orbital diagrams Orbital box diagrams of all elements are mentioned in the chart given below. Must contain at least 4 different symbols. It is found in the gas state at room temperature.
Carbon silicon and germanium germanium like silicon is also a semiconductor have a unique property in their electron structure -- each has four electrons in its outer orbital. Sulfur dioxide IUPAC-recommended spelling or sulphur dioxide traditional Commonwealth English is the chemical compound with the formula S O 2It is a toxic gas responsible for the smell of burnt matchesIt is released naturally by volcanic activity and is produced as a by-product of copper extraction and the burning of sulfur-bearing fossil fuels. So the remaining one electron will enter the 5s orbital in the clockwise direction.
Studies on troponin are widely increasing due to its main importance as a cardiac marker in ischemic heart diseasesIn. Unbinilium and Ubn are the temporary systematic IUPAC name and symbol which are used until the element is discovered confirmed and a permanent name is decided uponIn the periodic table of the. The 3d orbital is now full.
N 2 3F 2 2NF 3. With our money back guarantee our customers have the right to request and get a refund at any stage of their order in case something goes wrong. Carbon makes up only about 0025 percent of Earths crust.
Three isotopes occur naturally 12 C and 13 C being stable while 14 C. Water is the chemical substance with chemical formula H 2 O. They are potassium K calcium Ca gallium Ga germanium Ge arsenic As selenium Se bromine Br and krypton Kr.
Hunds principle is that electrons in different orbitals with the same energy would be positioned in such a way that they could be in the unpaired state of maximum number and the spin of the unpaired electrons will be one-way. Sulfur dioxide has a pungent. It is nonmetallic and tetravalentits atom making four electrons available to form covalent chemical bondsIt belongs to group 14 of the periodic table.
Figure 213 Electron Filling Diagram for the Element Iron Fe. All these elements have a valency of 2. Individual solar cell devices are often the.
Troponins are important protein molecules involve in muscle contraction. In the following diagram of a wave A a is amplitude and b is wavelength D a is amplitude and b is frequency B a is frequency and b is amplitude E a is wavelength and b is amplitude C a is wavelength and b is frequency Ans. Is there a main group element in period 4.
P 4 F 2. ASCII characters only characters found on a standard US keyboard. Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour.
Lewis is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adductA Lewis base then is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to. Browse our listings to find jobs in Germany for expats including jobs for English speakers or those in your native language. It is a form of photoelectric cell defined as a device whose electrical characteristics such as current voltage or resistance vary when exposed to light.
A solar cell or photovoltaic cell is an electronic device that converts the energy of light directly into electricity by the photovoltaic effect which is a physical and chemical phenomenon. One molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. Using the figure below categorize.
FeSe is a candidate system in which the presence of the lower-Hubbard band establishes the important role of electronic correlations orbital-dependent band shifts in the nematic phase and Fermi surface shrinking 710The strength of these effects can be suppressed by isoelectronic substitution with sulfur 1113The interatomic Coulomb. 6 to 30 characters long. Unbinilium also known as eka-radium or simply element 120 is the hypothetical chemical element in the periodic table with symbol Ubn and atomic number 120.
This graph is a fascinating object of study. The lefthand diagram shows the partial filling of the electron orbitals during the assembly of an iron atom. As oxygen belongs to group 16 6A or VIA along with sulfur S arsenic As and selenium Se tellurium Te polonium Po and livermorium Lv.
In the air carbon dioxide is transparent to visible light but absorbs infrared radiation acting as a greenhouse gasIt is a trace gas in Earths atmosphere at 417. A metalloid is a type of chemical element which has a preponderance of properties in between or that are a mixture of those of metals and nonmetalsThere is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Since energy must be conserved the energy difference between the two states equals the.
Carbon dioxide chemical formula CO 2 is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. There are a total of 8 main group elements in period 4 of the periodic table. Fluorine atoms react with Group-15 elements to form tetrachloride and pentachloride compounds.
Study with Quizlet and memorize flashcards containing terms like 71 1. These group elements are also called chalcogens. The outermost shell in the Bohr diagram of Chlorine contains 7 electrons that also called valence electrons.
Despite the lack of specificity the term remains in use in the literature of chemistry. The arsenic element in period 4 of the periodic table has five valence electrons. To create an orbital diagram of an atom you first need to know Hunds principle and Paulis exclusion principle.
The key difference between troponin I and troponin T is that the troponin I binds with actin while the troponin T binds with tropomyosin during muscle contractions. The 4p orbital is now full. It has several hundred million nodes today over a billion links and appears to grow exponentially with time.
Carbon from Latin carbo coal is a chemical element with the symbol C and atomic number 6. This allows them to form nice crystals. A Lewis acid named for the American physical chemist Gilbert N.
In physics emission is the process by which a higher energy quantum mechanical state of a particle becomes converted to a lower one through the emission of a photon resulting in the production of lightThe frequency of light emitted is a function of the energy of the transition. This can easily be observed in a.
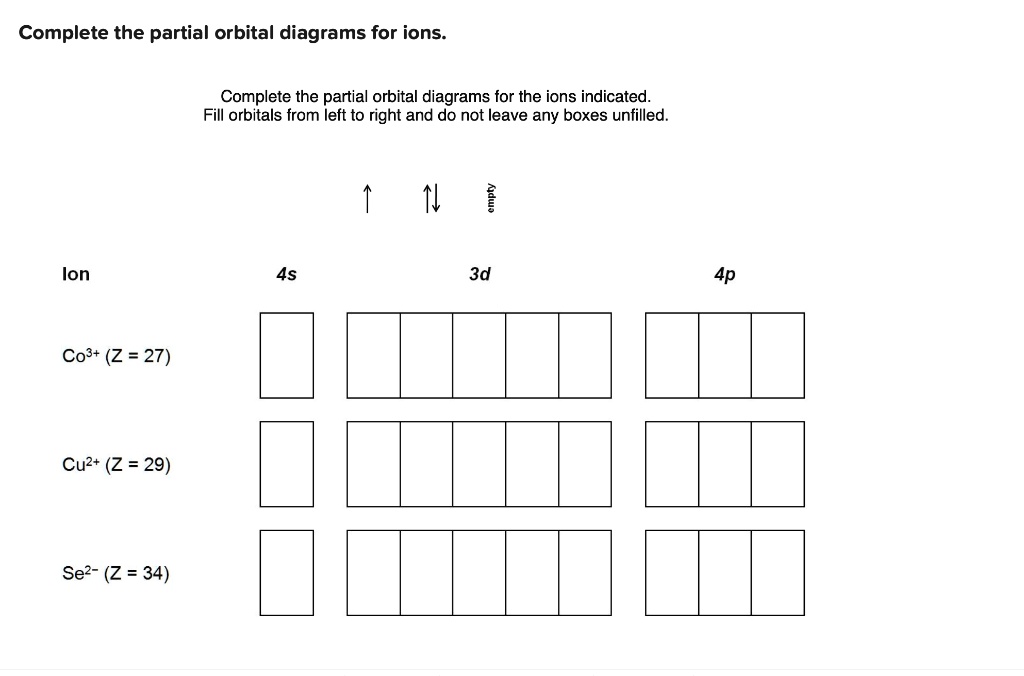
Solved Complete The Partial Orbital Diagrams For Ions Complete The Partial Orbital Diagrams For The Ions Indicated Fill Orbitals From Left To Right And Do Not Leave Any Boxes Unfilled Ion 4s
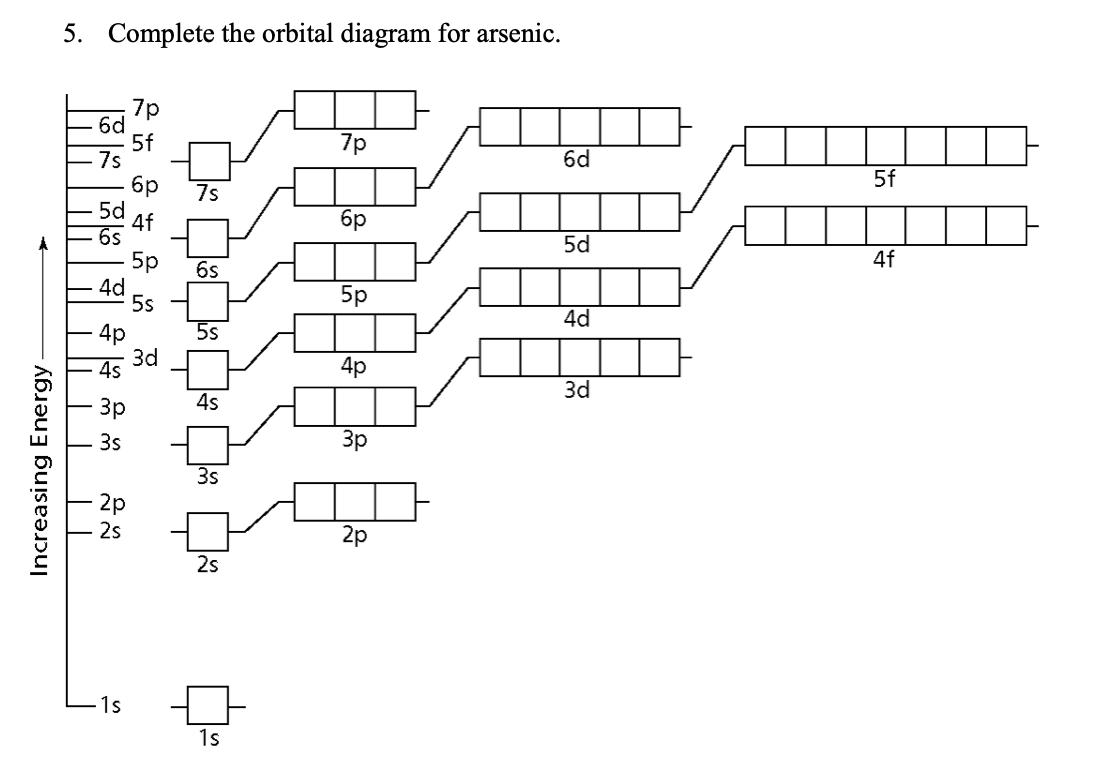
Solved 5 Complete The Orbital Diagram For Arsenic 7r 6d 5f Chegg Com
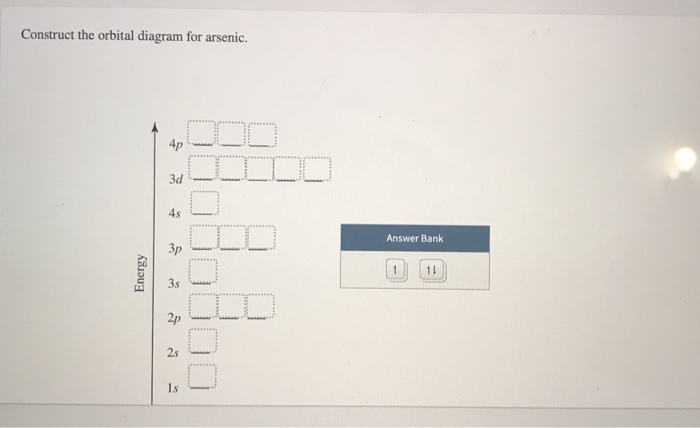
Solved Construct The Orbital Diagram For Arsenic 4p Lid 3d Chegg Com

Draw And Explain The Orbital Diagram For Titanium Homework Study Com
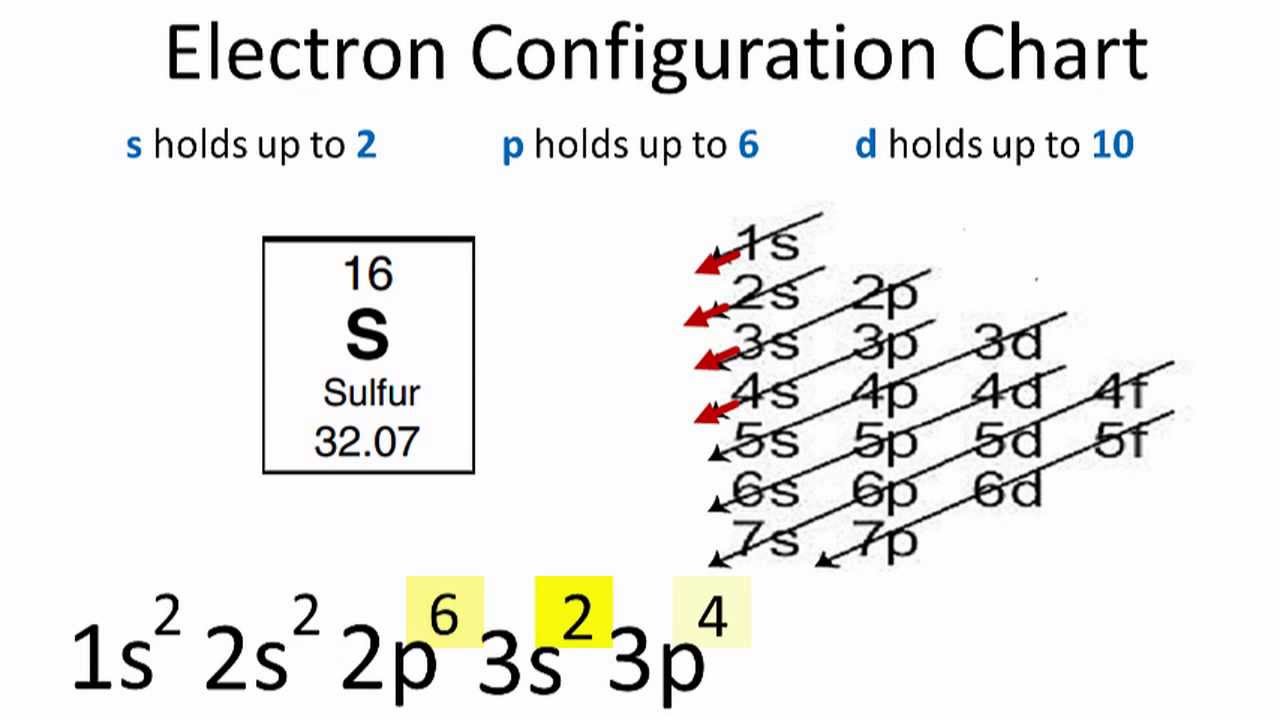
Sulfur Electron Configuration Youtube
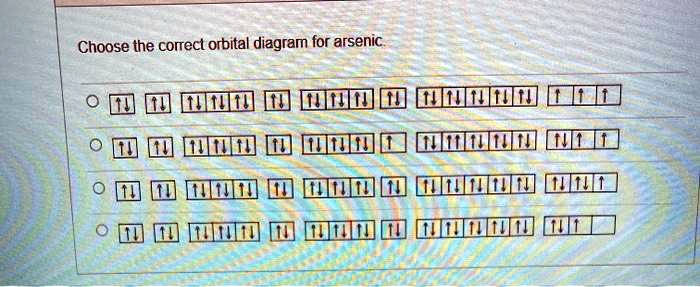
Solved Choose The Correct Orbital Diagram For Arsenic

Media Portfolio
Why Is The Molecular Orbital Diagram For O Different From N Quora
Strontium Atom
1643152520 585413 Png

Solved Question 7 Consider The Following Orbital Diagram 1s Chegg Com

4 Homo And Lumo Energy Levels In The Arsenic Molecule With Mp2 Levels Download Scientific Diagram

How To Write The Atomic Orbital Diagram For Arsenic As Youtube

How To Write The Atomic Orbital Diagram For Arsenic As Youtube

File Electron Configuration Bromine Svg Wikimedia Commons

File Electron Configuration Bromine Svg Wikimedia Commons
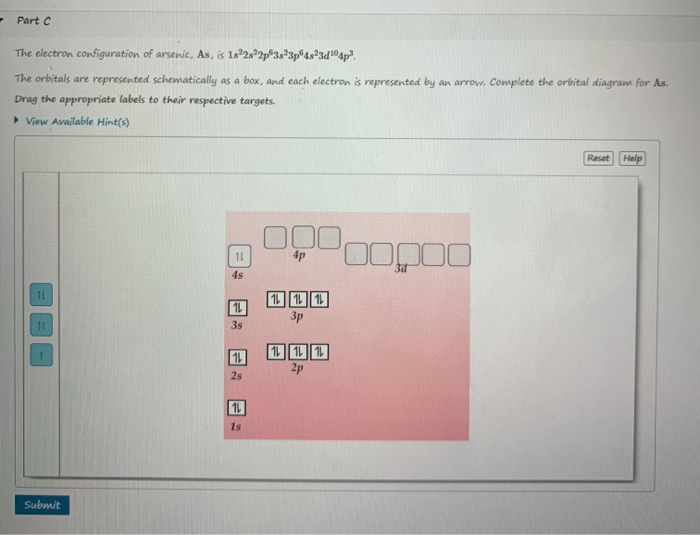
Solved Part C The Electron Configuration Of Arsenic As Is Chegg Com